Fertility Preservation Oncofertility
Cancer is the second most common cause of death among European Union residents. According to data from the National Cancer Registry, cancer incidence among women in Poland will increase by 15.6% by the end of 2025, reaching 99,000 cases. According to the National Cancer Institute, 10% of patients diagnosed with invasive cancer are women under 45 years old. Thanks to early diagnosis and the use of the latest oncological therapies, over 88% of women survive for more than 5 years. It was found that 54% of women of reproductive age diagnosed with cancer expressed a desire to become pregnant after completing oncological therapy.
The use of radio and chemotherapy in systemic therapy shows iatrogenic effects on gametes. It was previously thought that a dose of 4Gy leads to the loss of half of the oocytes. However, newer studies show that a dose below 2Gy is already sufficient to reduce ovarian reserve by half. Furthermore, it was found that radiotherapy results in endometrial atrophy and reduction in uterine muscle volume. Egg cells are very sensitive to chemotherapy, especially to alkylating agents. The use of aggressive chemotherapy in 80% of cases leads to damage to ovarian follicles and the oocytes they contain, consequently leading to premature ovarian insufficiency (POI) and loss of fertility.
For patients before oncological treatment in the reproductive period, as well as for girls before puberty, protocols have been developed to preserve reproductive health – oncofertility. According to current medical knowledge, the gold standard in fertility preservation for oncological patients is freezing oocytes or embryos. Starting a stimulation cycle is possible at any point in the cycle, without loss in terms of quantity or quality of obtained oocytes. It has also been shown that there is no delay in starting oncological therapy in patients undergoing the oncofertility procedure. On average, 71 days elapse from diagnosis to the inclusion of chemotherapy in patients undergoing the fertility preservation procedure versus 67 days in the group not covered by fertility protection. For patients with estrogen-dependent cancer, protocols have been developed using aromatase inhibitors and antiestrogens in adjuvant therapy. Another therapeutic option is to collect a fragment of ovarian tissue for cryopreservation and subsequent autotransplantation after completion of oncological treatment. This procedure not only allows for the restoration of reproductive function in patients after oncological treatment, but is the only one that also restores the hormonal function of the ovary.
We have been dealing with fertility preservation in oncological patients since 2015. So far, we have collected ovarian tissue from 107 women before starting systemic treatment (chemotherapy or radiotherapy) for freezing and subsequent autotransplantation.
The largest group of women were patients with breast cancer: 79%. The second group in terms of oncological diagnosis were patients with Hodgkin Lymphoma: 12%.
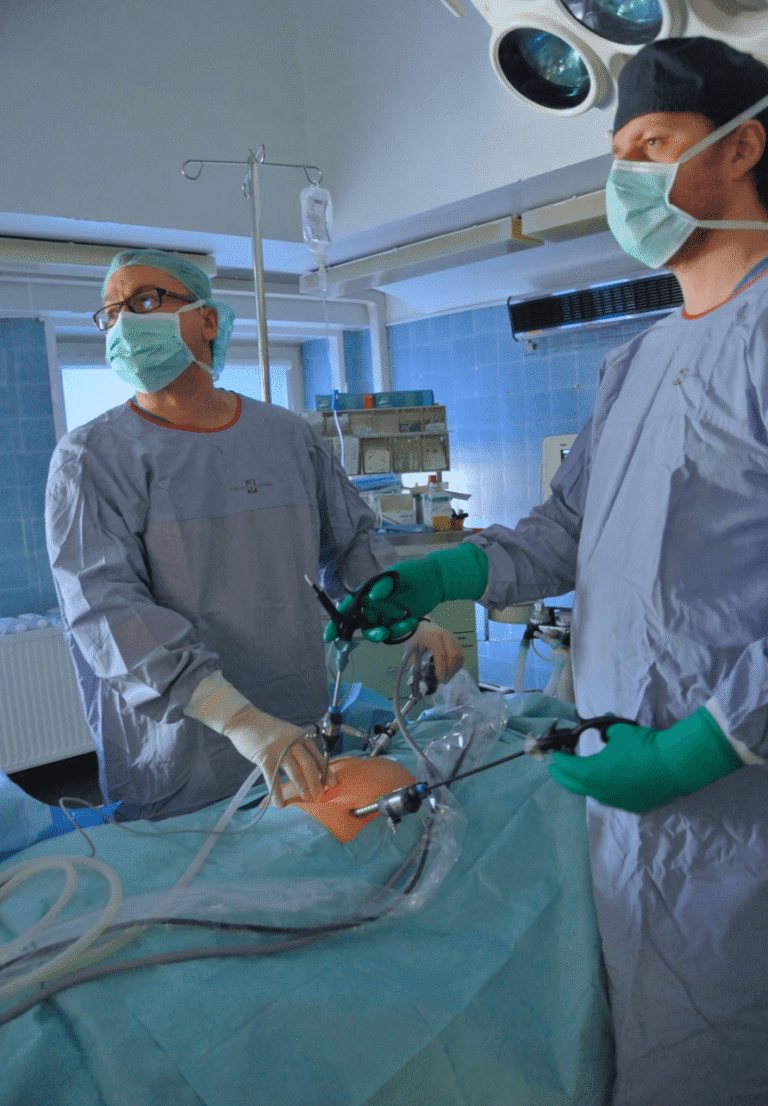
We conduct surgical procedures via microlaparotomy and laparoscopy (LPSC).
According to the procedure developed by Prof. Dror Meirow, we collect a fragment of ovarian tissue from only one gonad in all operated patients. The second non-operated ovary served (and in the case of women not yet operated on – will serve) as a site with unchanged blood supply for autograft acceptance. After transferring the ovarian tissue fragment to the embryology team, it was prepared for cryopreservation with histopathological control for oncological purity.
None of the operated women experienced complications after the applied therapy.
After obtaining the consent of the attending oncologist, the patient can proceed with autotransplantation.
The first patients who had previously had ovarian tissue collected underwent microlaparotomy, and ovarian tissue fragments were deposited subcapsularly and subperitoneally.
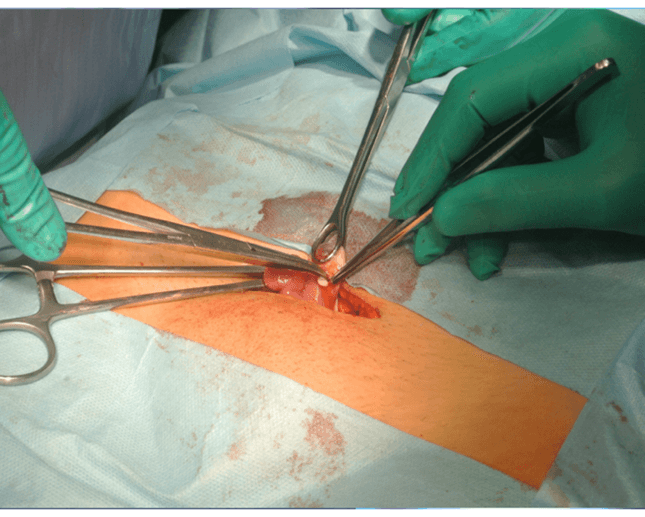
The time needed for the autograft to take was up to 6 months.
The use of LPSC for autotransplantation with simultaneous administration of growth factors (PRP) shortened the time for transplanted tissues to take to 3 weeks.
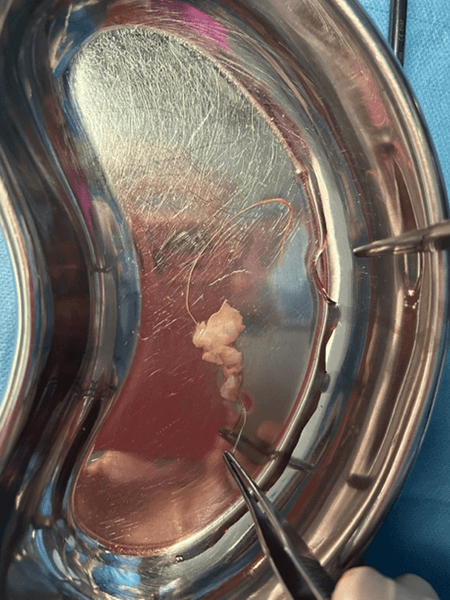
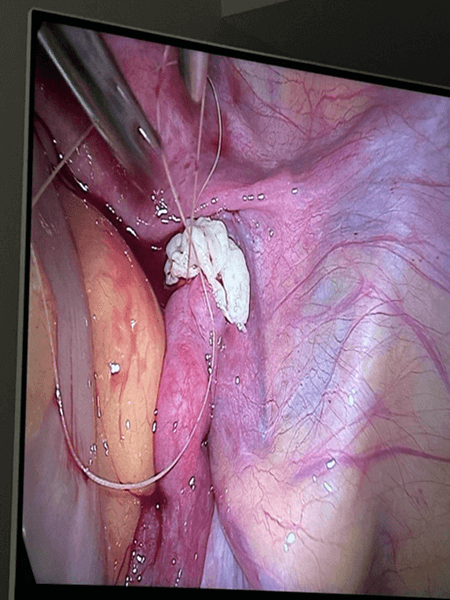
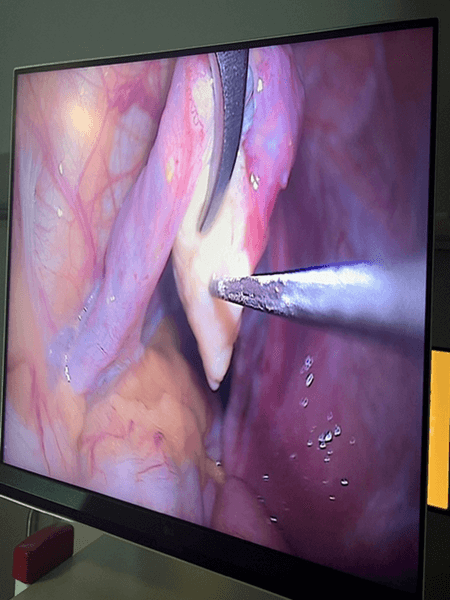
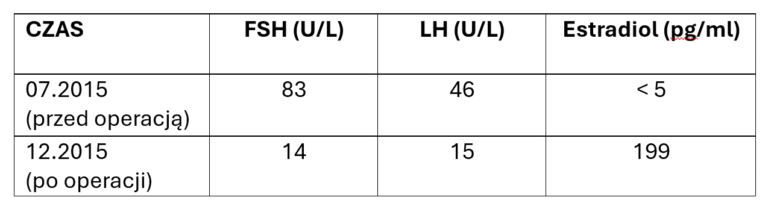
The return of ovarian function was monitored both in laboratory tests and in TV ultrasound examination.
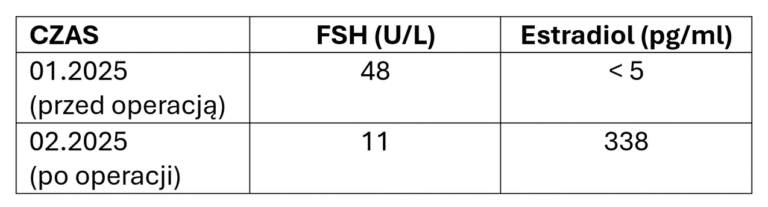
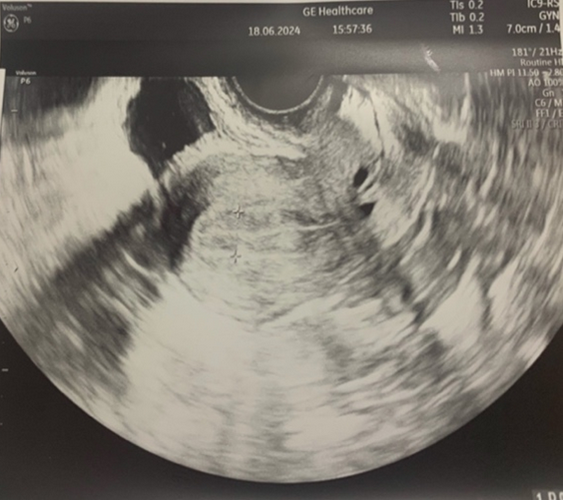
After obtaining a normal hormonal profile, patients wishing to become mothers undergo in vitro fertilization at an infertility treatment clinic.
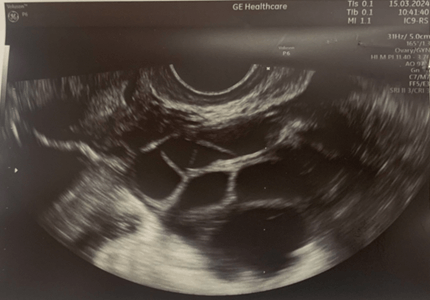
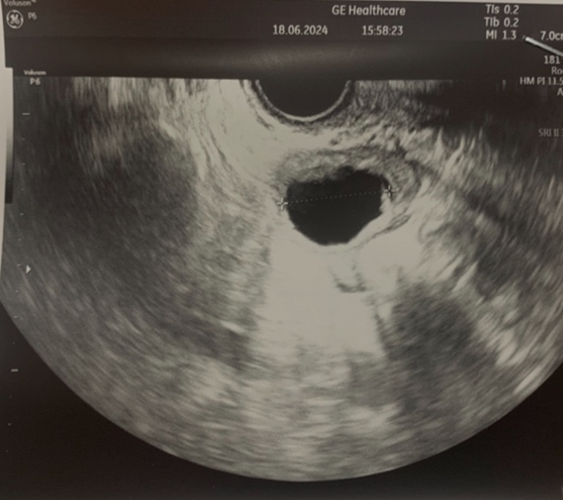
Ultrasound image of the left ovary: status post autotransplantation
of ovarian tissue fragments during controlled ovulation stimulation.
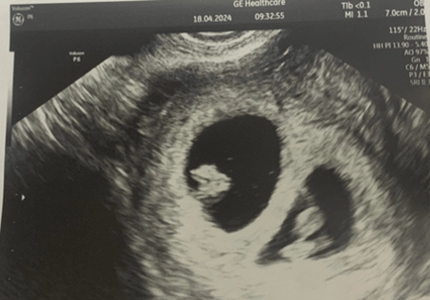
Ultrasound image of twin pregnancy at 6 weeks gestation, status post
transfer of 2 embryos.
