Ovarian tissue regeneration
Premature ovarian insufficiency (POI) affects 1% to 3.7% of women of reproductive age. This condition is characterized by the occurrence of typical menopausal symptoms below the age of 40, with a simultaneous increase in FSH and undetectable serum AMH levels. Considering that menopause in the Caucasian population occurs on average at 51.4 years, this is a significant shortening of the biological function of ovaries as endocrine glands in humans. Currently, there are two main theories explaining the etiology of the disease: genetic factors responsible for premature depletion of ovarian reserve, with an associated increased risk of cancer and shortened lifespan; autoimmune diseases – when antibodies against endocrine glands penetrate the follicular fluid and damage oocytes. The treatment for patients with POI is hormone replacement therapy. For patients who want to become mothers, oocyte donation is the treatment of choice.
Mesenchymal stem cells are multipotent cells that have the ability to differentiate and mature into cartilage, bone, and fat cells, and can help and interact with other stem cells. They also have the ability to modulate certain elements of the immune system. Mesenchymal stem cells are characterized by the ability to self-renew and differentiate into cartilage, bone, and fat, to adhere and grow in vitro. Mesenchymal stem cells can have different origins. Embryonic mesenchymal stem cells, fetal stem cells – from the umbilical cord and Wharton’s jelly, and adult mesenchymal cells from bone marrow and adipose tissue can be distinguished.
Mesenchymal stem cells derived from Wharton’s jelly meet the criteria described above: they are self-renewing and can differentiate into various tissues, not only bones, cartilage, and fat, but also into:
- striated muscle cells,
- cardiomyocytes,
- hepatocytes,
- pancreatic Langerhans cells,
- nerve cells.
They can also participate in the regeneration of retinal structures. On their surface, they have type I tissue compatibility antigens (HLA – I), but do not have surface markers of type II HLA antigens. The above immunological properties of these cells result in both a lack of immune response to mesenchymal cell transplantation from another donor and a lack of recognition by allogeneic mesenchymal cells of the recipient as foreign structures. It is believed that mesenchymal stem cells act as a local coordinator of tissue repair. Repair of damaged tissue occurs through the regulation of endogenous regenerative processes, rather than through the replacement of damaged tissue with de novo structures.
Fetal MSCs have a greater expansion potential in in vitro culture than adult mesenchymal stem cells. Many authors believe that this is a consequence of two passages of primary hematopoietic cells from the embryo to placental structures and back. First, between 4 and 12 days of embryogenesis, hematopoietic cells migrate from the yolk sac to the placenta through the primitive umbilical cord. Then, during the second migration, MSCs are transferred in the opposite direction. They return from the placenta back to the fetus: to the liver, and then to the bone marrow. Scientists have hypothesized that during this migration, mesenchymal cells in Wharton’s jelly become “trapped” from early embryogenesis, throughout pregnancy, until birth.
Recent discoveries made in the field of stem cell origin and development prove that primordial germ cells (PGCs) and their deposits, remaining in the developing tissues of the organism – very small embryonic-like stem cells (VSEL) – play a key role in the regeneration process. Some earlier studies suggest that primordial stem cells (very small MSCs deposited in the ovarian tract) undergo asymmetric cell divisions and are able to produce oogonial/germinal stem cells, which ultimately become oocytes. The factor stimulating vsMSCs to divide may be mesenchymal stem cells and the exosomes they produce. Animal models have shown that stimulation of primordial stem cells to produce oocytes is able to restore the hormonal and procreative function of ovaries previously damaged by radio- and chemotherapy. This suggests that similar benefits should be experienced by women who have developed POI.
DeCure
Own experiences
We have been conducting research using mesenchymal stem cells (MSC) in patients with premature ovarian insufficiency (POI) since 2016.
We have experience with using stem cells obtained from Wharton’s jelly, adipose tissue, and bone marrow.
We administer the biological material directly into the ovarian tissue during laparoscopy.
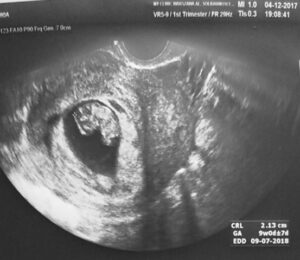
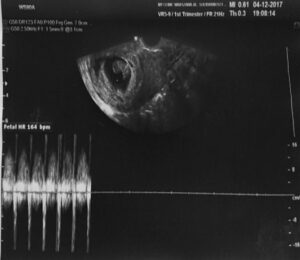
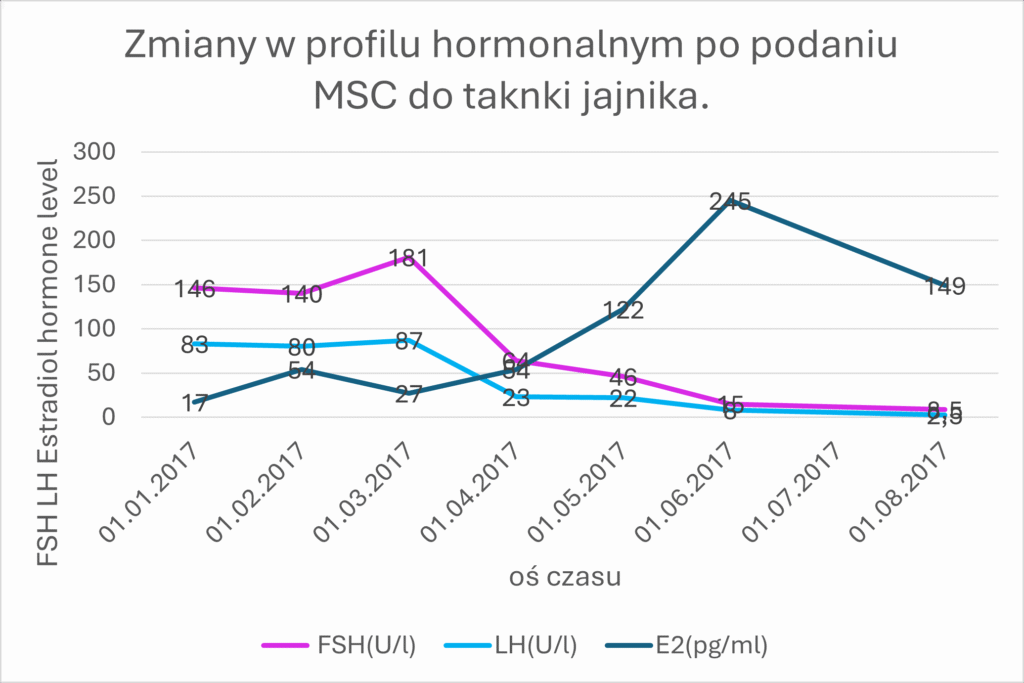
The use of MSCs from the umbilical cord allowed for the restoration of hormonal and procreative function in the ovarian tissue. We observe the biological effect measured as the concentration of sex hormones in the blood serum in patients after administration of biological material after a period of 3 to 6 months. The graph shows the results of a 35-year-old patient with premature menopause (FSH 146 U/l, LH 83 U/l, E2 17 pg/ml). After the administration of stem cells, there was a return to normal sex hormone values (FSH 2.5 U/l, E2 149 pg/ml)
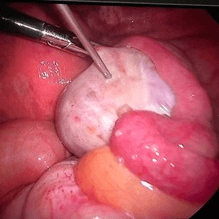
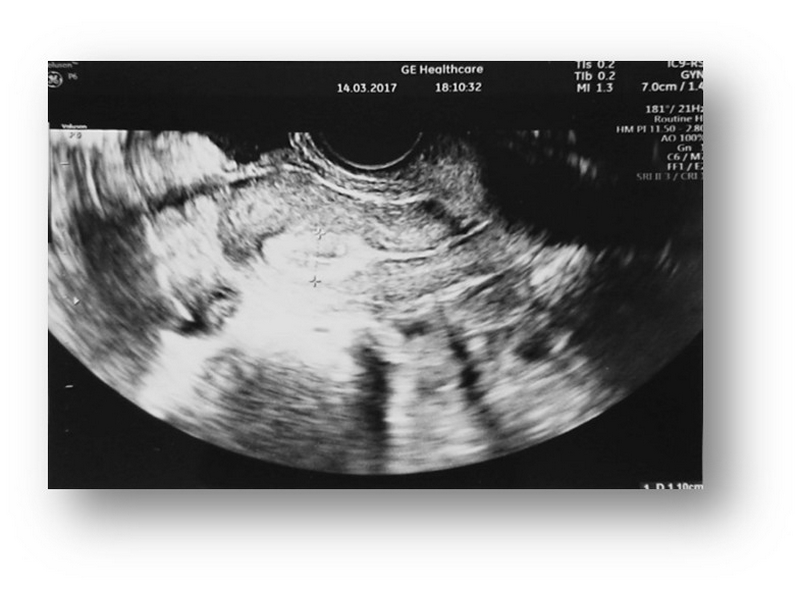
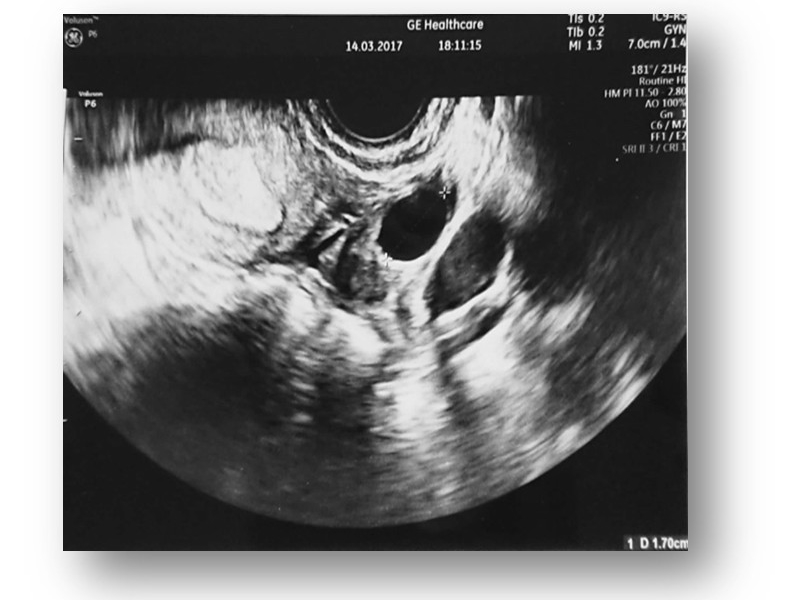
Changes are also observed during transvaginal ultrasound examination.
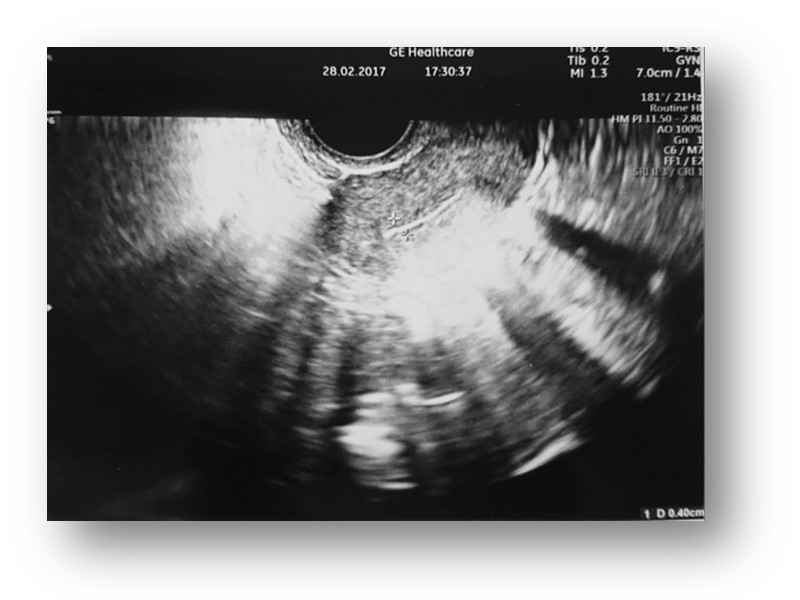
TV ultrasound image of the uterus with endometrium and ovary before MSC administration.
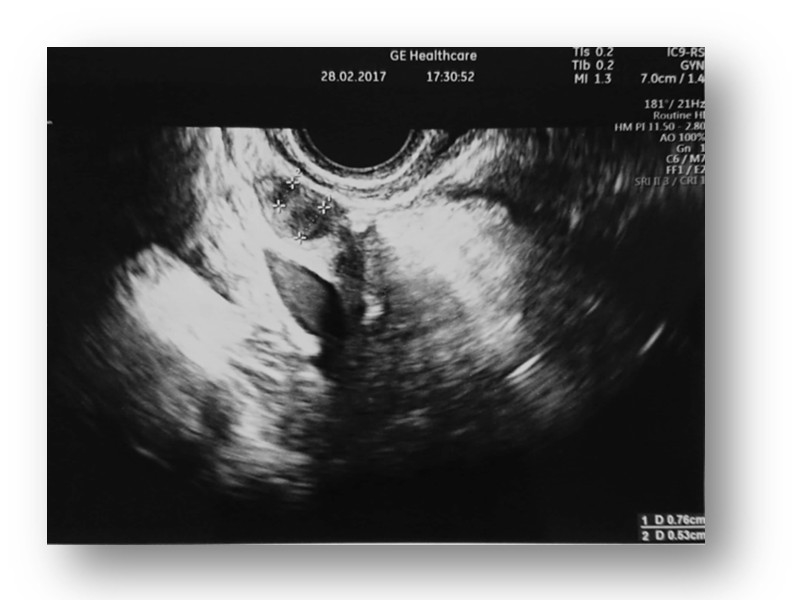
TV ultrasound image of the uterus with endometrium and ovary after MSC administration.
Patients have a chance to achieve pregnancy
both in the IVF program


Blastocyst – embryo on day 5 of life
and as a result of
spontaneous conception,